Completion requirements
Stereoisomers are isomers that differ from each other only in the relative spatial arrangement of their component groups or atoms, which remain identical. There are conformational stereoisomers that differ in their conformations, and configurational stereoisomers that differ in their configurations.
III-1 Configurational Stereoisomers
Configurational stereoisomers are stereoisomers that differ in their configurations.
III-1-1 Configuration
The fixed and relative arrangement of an atom's substituents in space defines its configuration. This term applies more
specifically to at least trivalent atoms with substituents that are all different from each other and that can create a
chiral center
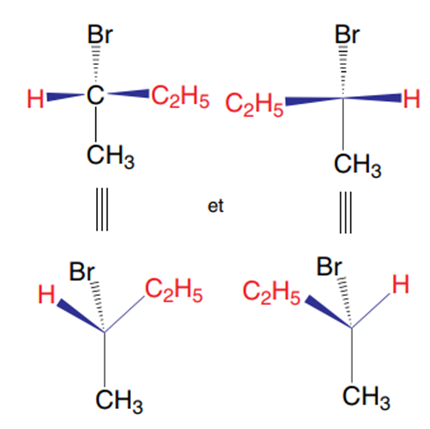
pairs of configurational stereoisomers
III-1-2 Asymmetric Carbon or Chiral Carbon
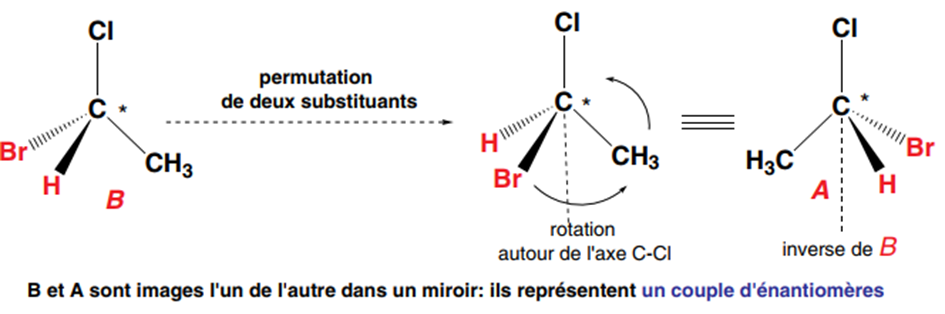
A carbon that carries four different substituents is asymmetric or chiral. Properties: When a carbon (A) is asymmetric, its mirror image or its symmetrical shape about a plane (B) cannot be superimposed on it. Itself (A) and its inverse (B) about a plane represent two inverted configurations of the molecule. This is a pair of enantiomers. They are mirror images of each other (we also say that B is the mirror image of A, and vice versa).
exp
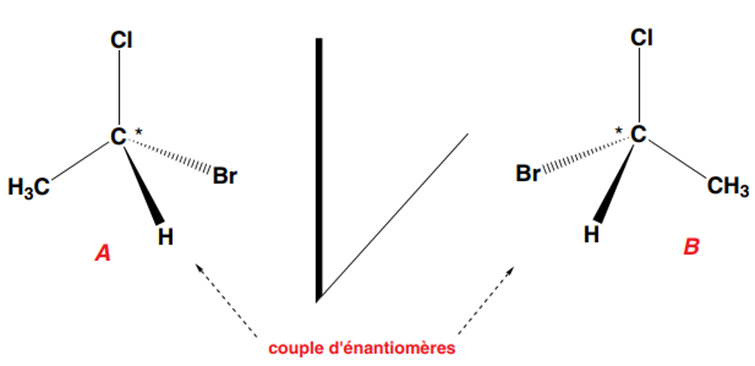
The exchange of two substituents of one of the two enantiomers (here B), which requires breaking two C-Br and C-H bonds and re-establishing new C-H and C-Br bonds, transforms it into the other enantiomer (A), thus inverting its configuration.
Ø The molecule which has an asymmetric carbon, symbolized by C*
III-1-3-Absolute Configuration (R and S Nomenclature)
To define the configurations of asymmetric carbons in molecules, three chemists, Robert S. Cahn, Christopher Ingold, and Vladimir Prelog, defined rules allowing these carbons to be classified according to their so-called absolute configuration into the R series (from the Latin rectus, on the right) and the S series (from the Latin sinister, on the left). The rules to apply to achieve this are as follows:
1. Order the substituents of the asymmetric carbon according to their order of priority by applying the rules described to define the E and Z isomers of alkenes.
2. Then, look at the triangle formed from the substituents classified 1, 2, and 3 according to their order of priority, such that the fourth substituent is located behind the plane of this triangle,
relative to the observer. When the rotation is clockwise (to the right), the carbon is said to be of absolute R configuration. Otherwise, to the left, the asymmetric carbon is said to be of absolute S configuration.
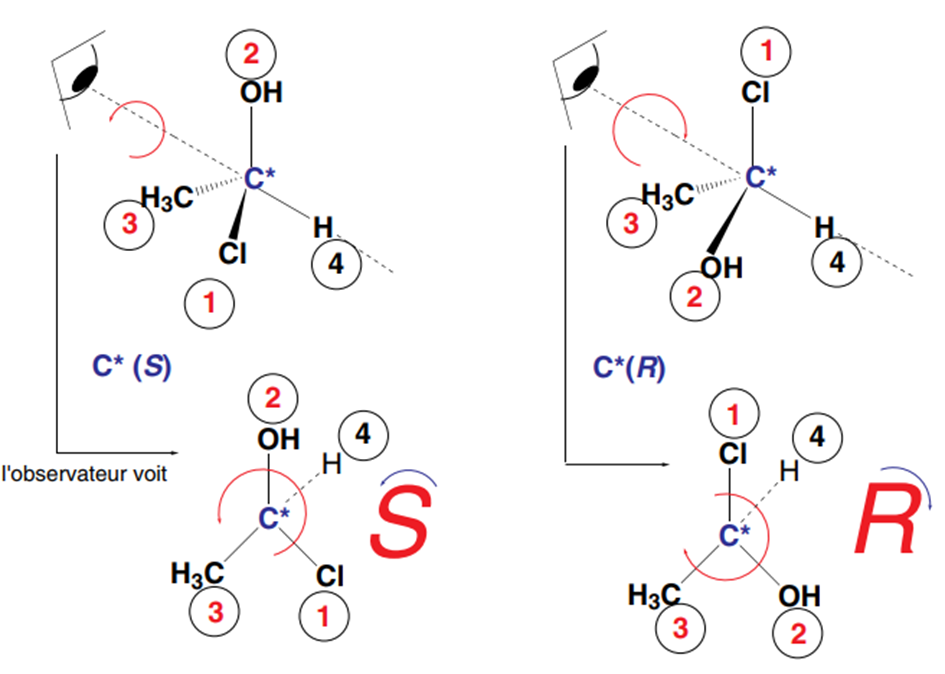
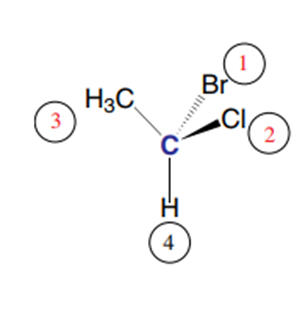
If the 1,2,3 triangle is viewed from the side opposite the hydrogen atom to meet the conditions of the Cahn, Ingold, and Prelog rule, we see:
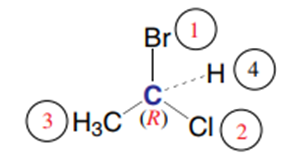
and the asymmetric carbon has the absolute configuration R
Exp2
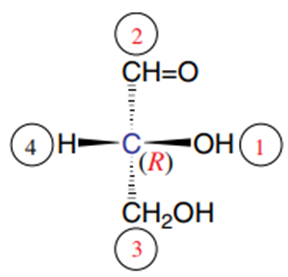
In this representation of (D)-glyceraldehyde, the direction of rotation 1 2 3 is counterclockwise, but note that substituent 4 is at the front of the representation, i.e., in front of the 1,2,3 triangle, thus in the opposite direction to the Cahn, Ingold, and Prelog rule. It is therefore not an asymmetric S carbon but, on the contrary, an asymmetric R carbon.
III-2-Conformational Stereoisomers (Conformers or Rotamers)
Molecules that can form various stereoisomers by simple rotation(s) around their single V bonds are called "flexible molecules": the different spatial structures that result are called conformations. Two stereoisomers with different conformations are called conformers or rotamers.
Numerous electrostatic interactions exist between the atoms of a molecule due to their electron densities), steric hindrances linked to large atoms such as chlorine, bromine, branched alkyl groups such as the t-butyl group -C(CH3)3, etc.). These interactions repel or attract atoms depending on their charges and/or volumes, making the transition from one conformation to another sometimes difficult: it is said that there are potential barriers that favor certain conformations of the molecule, called stable. These correspond to the lowest molecular potential energy values. To move from one conformation to another, these potential barriers must be crossed by adding energy to the molecule (by heating or ultraviolet radiation). There are therefore preferred conformations that are more stable than others, at 25 qC, which will, a priori, be the most prevalent.
III-2-1-Newman Representation
Considering the projective representation of ethane, we see that by rotating carbon 2, or C-2, relative to carbon 1, or C-1, it is possible to obtain an infinite number of conformations. Only two of these are studied because they correspond to different levels of the molecule's potential energy, with potential barriers or interconversion barriers being crossed. The multitude of other possible conformations are grouped under the name oblique conformations.
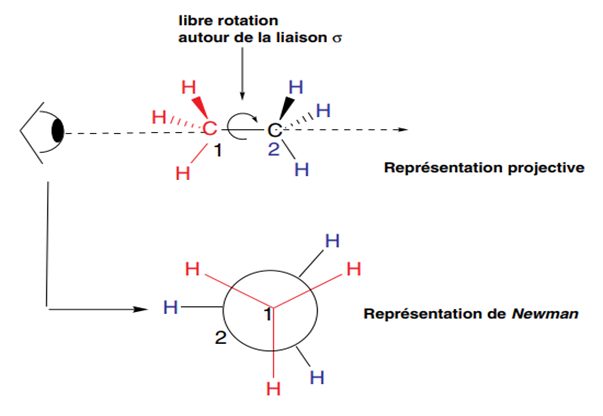
To easily draw conformers, the Newman representation is used. It consists of drawing what an observer (represented by an eye) sees when they place the molecule in front of them along the C-1-C-2 bond axis. They then see three 120° bonds for the three substituents of C-1, and the same for C-2, this second carbon being symbolized in this representation by a circle for clarity. When C-2 bonds are hidden by those of C-1 (eclipsed conformations), the lines are slightly offset to facilitate drawing. Figure 2 shows the various conformers of n-butane chosen according to the criteria indicated above. They are obtained by rotating carbon 2 several times by 60° relative to carbon 1 around the C1-C2 bond.
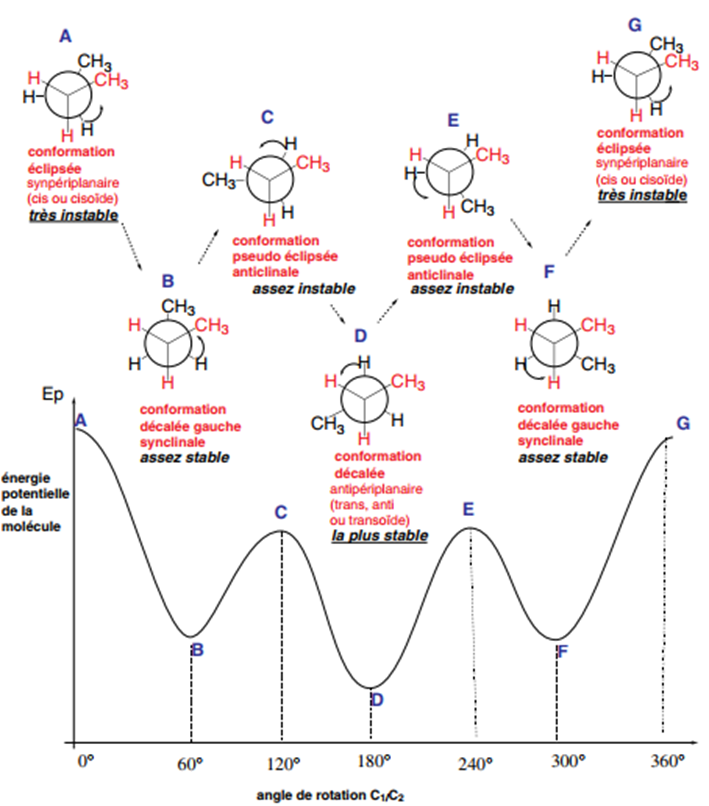
The 6 most important conformers of butane are shown in Figure 2. In the eclipsed conformation (also called synperiplanar, cis or cisoid) (A), the largest substituent of C-1 eclipses the largest substituent of C-2. The distance between the two groups is the smallest of all conformations and if the groups are of the same electrostatic nature, they repel each other because the interactions are very strong. There can also be steric hindrance if the two groups are large, and the conformation is very unstable: the potential energy of the molecule is then the highest as shown by the curve Ep = f (rotation angle C-1/C-2).
On the other hand, among the three shifted conformations, anti (D) and left-shifted
synclinal (B and F), more stable than the eclipsed conformations, the one in which the two methyl groups, the largest of the two carbons, are as far apart as possible, is the most stable: it corresponds to the minimum potential energy of the molecule
(weakest interactions between bulky groups, due to their distance).
• Sometimes, intramolecular interactions between two functional groups can stabilize
conformations that are a priori considered unstable. This is the case for 1,2-diols or glycols, whose eclipsed conformation is relatively large due to the creation of a hydrogen bond between the H of one OH group, which has a partial positive charge, and the oxygen of the second OH group, which, on the contrary, has a partial negative charge in figure3
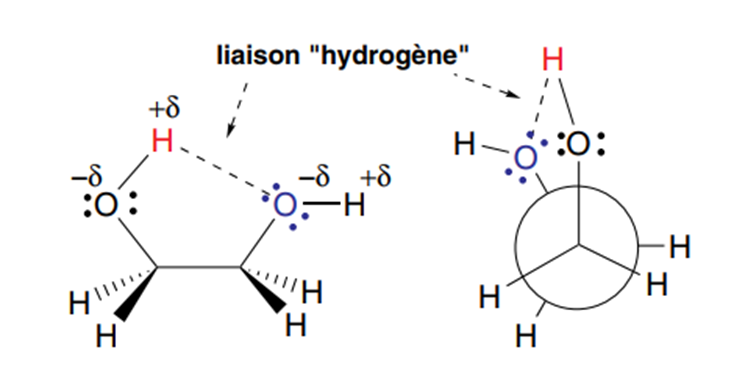
figure 3
For conformational analysis, if there are several different substituents on the two carbons considered, the conformer names are defined after searching for the substituent on each carbon that has priority according to the priority rule indicated for the determination of the geometric E or Z isomers of ethylenic derivatives. We then refer to what was indicated for the methyl groups (priority over hydrogens) of the conformers of n-butane, as in the following example in Figure 4.
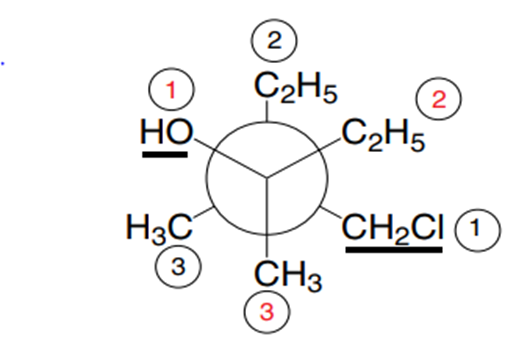
Figure 4
In this example, the two priority groups of each of the C-1 and C-2 carbons are in trans position relative to each other: this is therefore a shifted conformation, the most stable.
