Completion requirements
I INDUCTIVE (OR INDUCING) EFFECTS
a) Principle: The more electronegative atom will attract part of the electron cloud of the γ bond (attractive effect, denoted by -I), and the more electropositive atom will, on the contrary, donate part of the electron cloud of the γ bond (donor effect, denoted by +I).
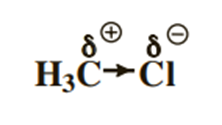
Ø MESOMER EFFECTS OR RESONANCE
This effect applies to π bonds, whether localized between two atoms or delocalized to form conjugated molecules, as well as to lone pairs adjacent to a double bond.
The terms "mesomeric effect" and "resonance" are synonymous; the former refers to the electronic structure aspect and the latter to the energy aspect.
Basic examples
1) Positive charge adjacent to a double bond
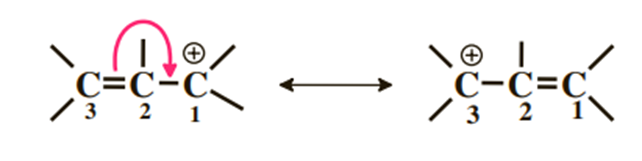
This result clearly shows that the positive charge is distributed over carbons 1 and 3 and that the π cloud is delocalized over the three carbons.
2) Negative charge adjacent to a double bond
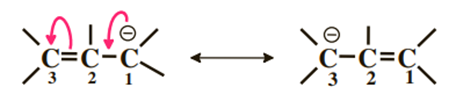
This time, it is the negative charge that is delocalized on carbons 1 and 3 and as before the π cloud is delocalized on the three carbons.
3) Single electron adjacent to a double bond
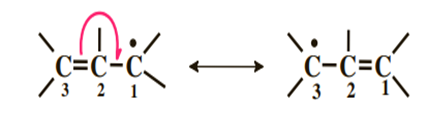
Note that we use half-arrows because, unlike in the previous cases, we only move one electron at a time. The single electron is distributed between carbons 1 and 3.
4) Atom with a pair adjacent to a double bond
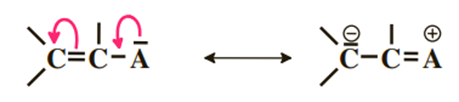
This case generalizes the example of vinyl chloride seen previously. There is delocalization of the doublet on the C–A bond. This delocalization is weak because in the second form there is a positive charge on an electronegative atom (A).
5) Case of two alternating double bonds
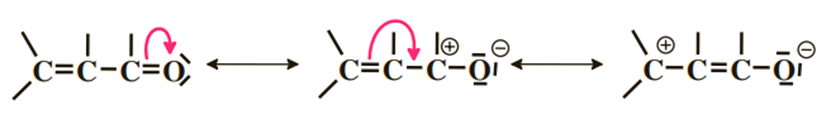
We have three limiting forms. We clearly show the delocalization of the π cloud throughout the chain and a localization of the negative charge on oxygen while the positive charges are distributed on carbons 1 and 3.
